September 2025 Issue

A Note From Our Chief Operating Officer
At PicnicHealth, our commitment to exceptional partnership and study delivery is at the heart of what we do. As we’ve expanded our collaborations and taken on new studies this year, we’ve also invested in growing our teams and deepening our expertise to better serve you.
This summer, we welcomed two outstanding leaders to our Delivery team: Katie Fox, Head of Delivery Success and Operations, and Lindsey Wahlstrom, Head of Patient Recruitment and Engagement. Katie brings a strong background in study delivery and is here to optimize our delivery processes and ensure a best-in-class customer experience as we scale. Lindsey brings extensive experience in rare disease patient advocacy and recruitment, helping us expand our recruitment channels and build a more effective recruitment engine.
Together with Kristina Cotter, our Head of Scientific Delivery, who has been instrumental in our study delivery over the past two years, these leaders are driving forward our commitment to excellence. Their combined expertise ensures that PicnicHealth continues to deliver the quality insights and seamless research experience you expect.
Please reach out at any time to your PicnicHealth team with any questions, concerns, or to discuss how we can help you reach your research objectives in 2026. We’re grateful for the trust you place in us and excited to continue delivering impactful studies together.
Luna Federici
Chief Operating Officer at PicnicHealth

What's New at PicnicHealth
Coming Soon: Our Mobile App!
In just a few weeks, our Patient Portal will be launching as a mobile app for iOS and Android!
This new, accessible extension of our portal empowers patients to take even more control of their health data journey and enables on-demand engagement with PicnicHealth wherever they are.
From filling out patient-reported outcomes to receiving real-time prompts and reminders for key study activities, our user-friendly app will make engaging in your study even easier.
The user-friendly app streamlines completion of study-related activities and makes medical records and the smart health assistant more accessible to patients in their care journey.
Patients can choose to use the web-based application and/or the mobile app, depending on user preference.
App notifications and reminders supports daily health management and helps patients remember to complete their study activities.
By making participation easier, we’re generating more robust, complete medical data to advance research.

Customer Spotlight
Prioritizing Patients: How Biogen and PicnicHealth Are Transforming Rare Disease Research
Biogen partnered with PicnicHealth to launch a rare disease drug registry supporting their newly approved therapy. Through this collaboration, PicnicHealth is enrolling patients through Biogen’s specialty pharmacy distributor and generating rich, real-world data by integrating medical records with patient-generated data. In this interview, we speak with Boyang Bian, Head of US Medical Evidence Generation at Biogen, to learn more about the partnership and its impact on advancing rare disease research.
Disclaimer: The views expressed in this Q&A are Boyang’s personal opinions and do not necessarily reflect those of Biogen.
What research questions is PicnicHealth helping you to explore?
PicnicHealth is helping us generate real-world evidence for one of our rare disease product indications. Our research is focused on understanding disease progression, treatment patterns, and importantly, the lived experience of patients with their condition and with our therapy. By combining curated medical records with patient-reported outcomes and wearable device data, we’re able to build a more holistic picture that allows us to answer questions we previously could not address.
Why did you choose PicnicHealth to answer your research questions?
I first learned about PicnicHealth while at a previous company and was immediately intrigued by your unique model and genuine commitment to patient-centricity. When we started the effort to explore this rare disease RWE study at Biogen, I reached out because we wanted a study partner that not only advanced scientific knowledge but also brought tangible value back to patients, helping them better understand their treatment journey.
Throughout our conversations, I was struck by the quality and depth of the curated data, as well as PicnicHealth’s ability to address research questions that are difficult to answer through traditional data sources. Your capability to directly engage with patients through a mobile app to administer PRO questionnaires—and integrate that feedback with wearable and clinical data—was exactly what we were looking for. On top of that, your strong track record of publications in rare disease research gave us added confidence in the partnership.
How do you see the conduct of non-interventional research evolving in the next few years?
The field is changing very fast. I’m particularly excited to see the growth of decentralized, patient-driven research models. Traditional site-based studies often work well for physicians and sites but not always for patients. Approaches that integrate digital health technologies, electronic medical records, AI, and patient-reported outcomes are making research more inclusive, efficient, and representative.
This evolution gives researchers like me new opportunities to generate meaningful insights that not only advance medical understanding but also directly empower patients. This is especially powerful for rare diseases, but its impact resonates across many other conditions, driving more precise, patient-focused evidence and, ultimately, better care.
PicnicHealth supports non-interventional research across portfolios, therapeutic areas, and research needs to build long-term partnerships that drive research forward. Have you explored how PicnicHealth could assist your colleagues with their research needs? Reach out to a member of your study team to discuss!
Product Corner
Ready, Willing, and Connected: The Patient Perspective on Remote Clinical Research
Are study participants ready to embrace digital health technologies?
Remote data capture technologies, from at-home diagnostic kits to consumer wearables, are reshaping how we gather real-world evidence and conduct clinical research. By combining isolated data points with more frequent, even continuous, streams of patient data, researchers can create a more complete picture of the patient journey for stronger evidence generation.
This transformation gained regulatory momentum when the FDA released its final guidance on "Digital Health Technologies for Remote Data Acquisition in Clinical Investigations" in December 2023, establishing a fit-for-use framework that prioritizes data integrity while embracing technological innovation. However, regulatory approval represents only half the equation. The success of research studies leveraging digital health technologies ultimately hinges on the willingness of participants to adopt these technologies.
A recent study by PicnicHealth addresses the critical market question: Are study participants ready to embrace these digital tools in non-interventional research? The research, “Leveraging Remote Data Capture to Generate Real-World Evidence for Clinical Transformation,” was based on an exploratory survey of 41 patients and caregivers already engaged in non-interventional studies to assess their willingness to adopt emerging data capture technologies.
Market Implications: Strong Patient Readiness Signals Growth Opportunity
The findings reveal overwhelmingly positive patient sentiment, indicating substantial market readiness for expanded digital health technology deployment. This patient acceptance creates a compelling business case for life sciences organizations to adopt these study insight-enhancing data sources.
Key Takeaways From the Study
Wearable Technology is Welcome: The use of wearable devices like smartwatches for health data collection is widely accepted. A remarkable 90% of participants would be willing to wear a device shipped to them for a study. In fact, 86% feel comfortable using a smartwatch or fitness tracker for this purpose.
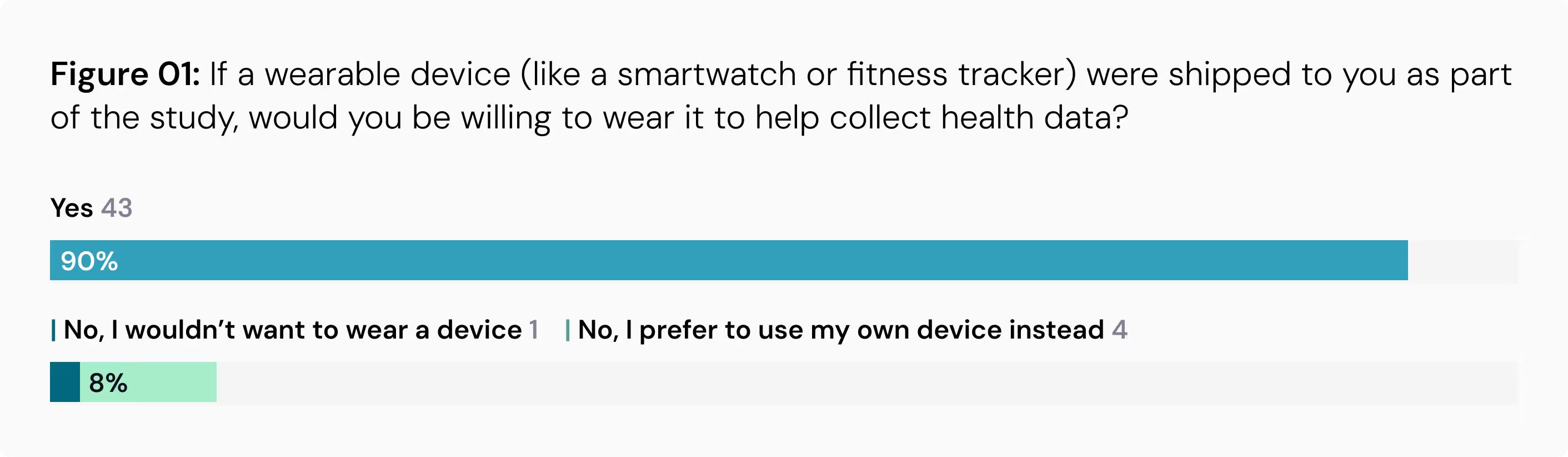
Televisits Are Favored: The study found that virtual appointments increase the likelihood of participation. More than half of the respondents (55%) said that having video or telehealth appointments instead of in-person visits would make them more likely to take part in a study.
At-Home Sample Collection is Preferred: When a small blood sample is needed, 47% of participants would prefer to collect it themselves using a mail-in kit rather than visiting a lab.

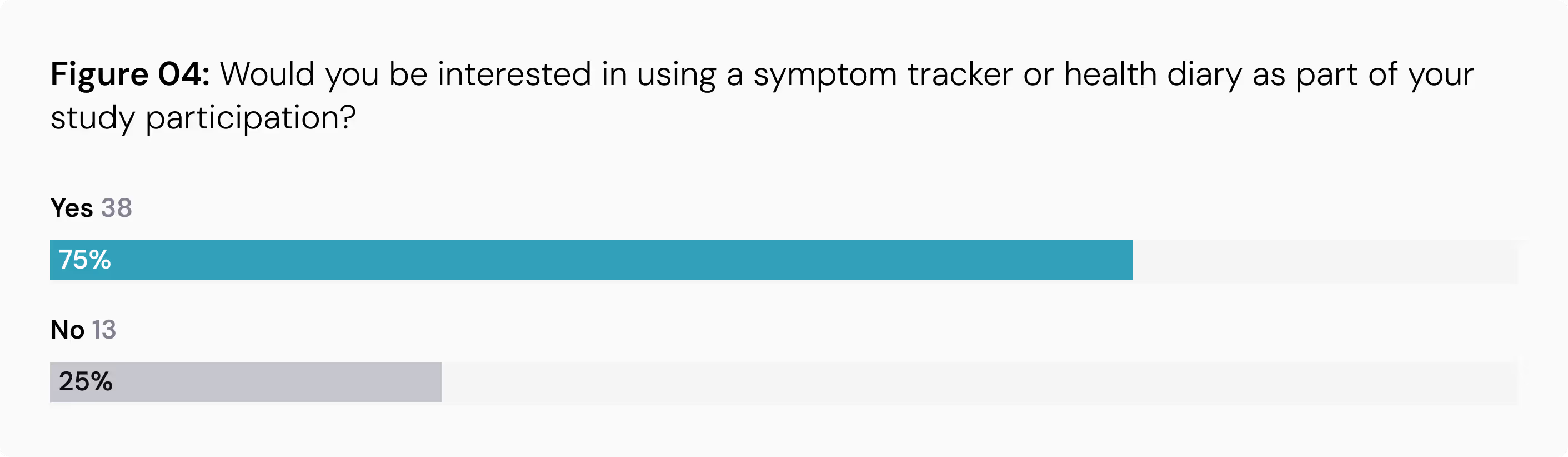
High Interest in Symptom Tracking: A significant 75% of participants expressed interest in using a symptom tracker or health diary as part of a study. Encouragingly, many are willing to log entries frequently, with 34% open to daily tracking and another 34% willing to do so a few times a week.
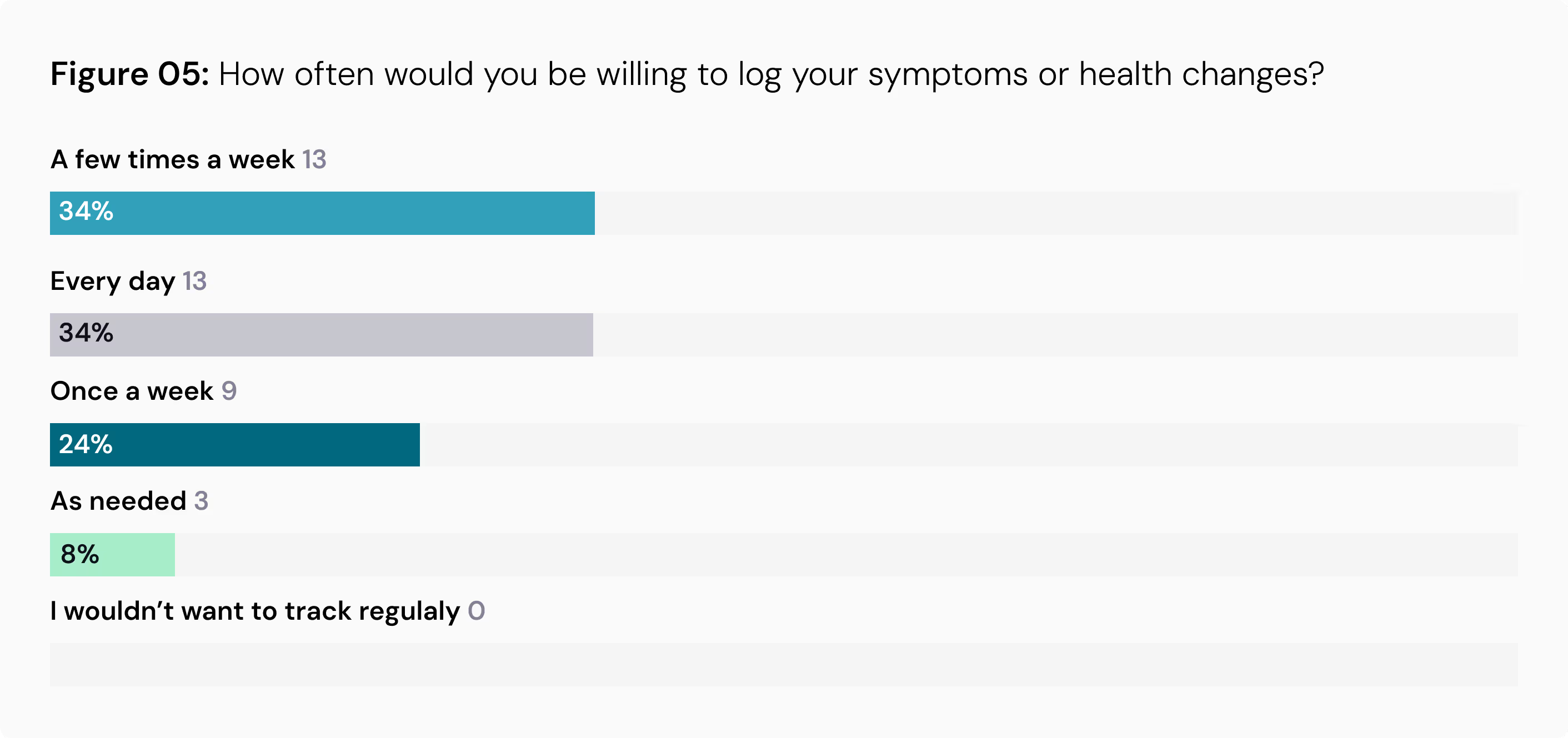
Patient Preferences for Data Capture Technologies
In addition to the willingness of study participants to adopt these emerging methods, the survey revealed patient preferences that can inform their successful implementation.
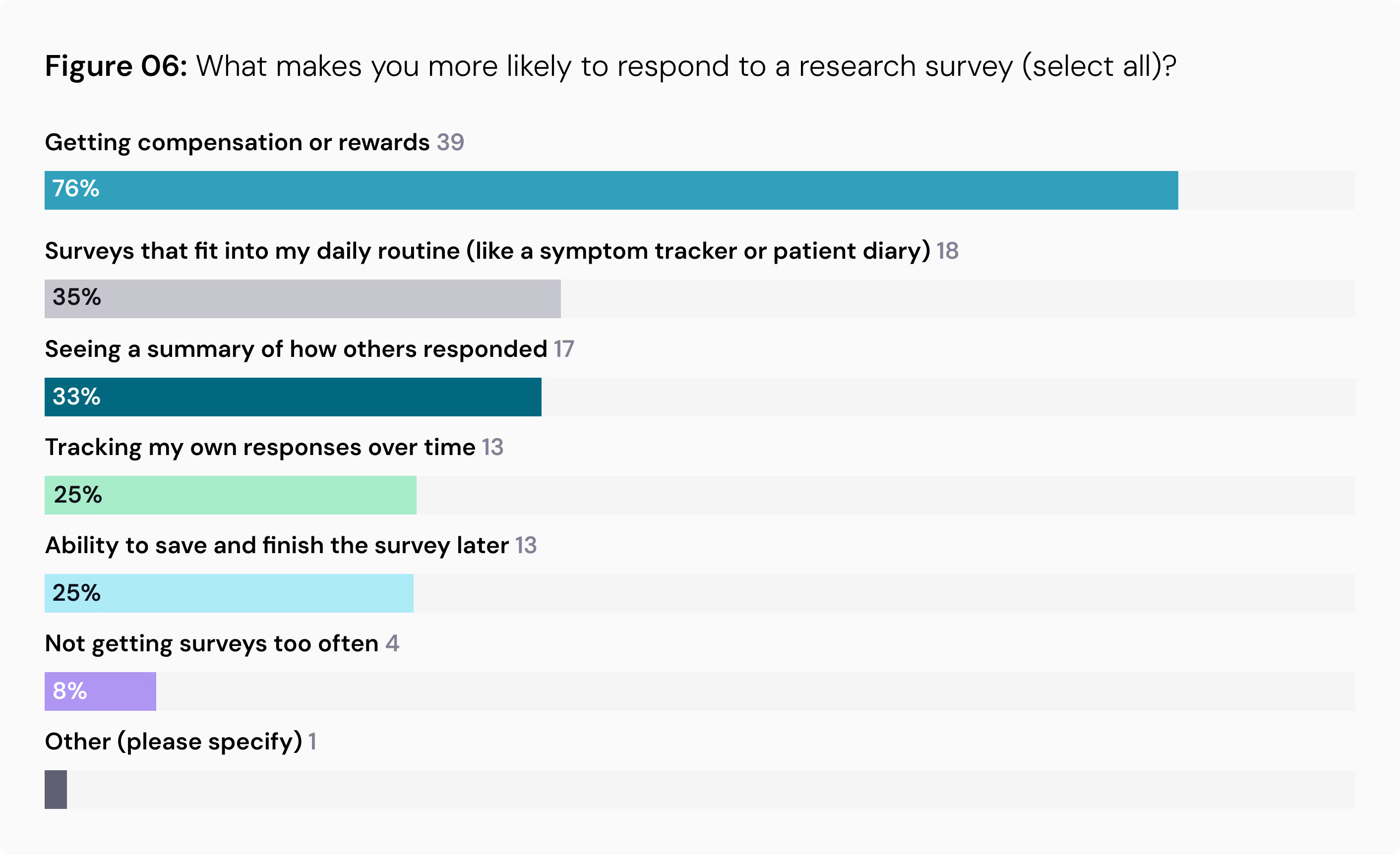
Convenience and Compensation Drive Participation: When asked what makes them more likely to respond to a research survey, 76% of respondents pointed to compensation or rewards. Convenience also ranked high, with 35% valuing surveys that fit into their daily routine and 25% wanting the ability to save and finish a survey later.
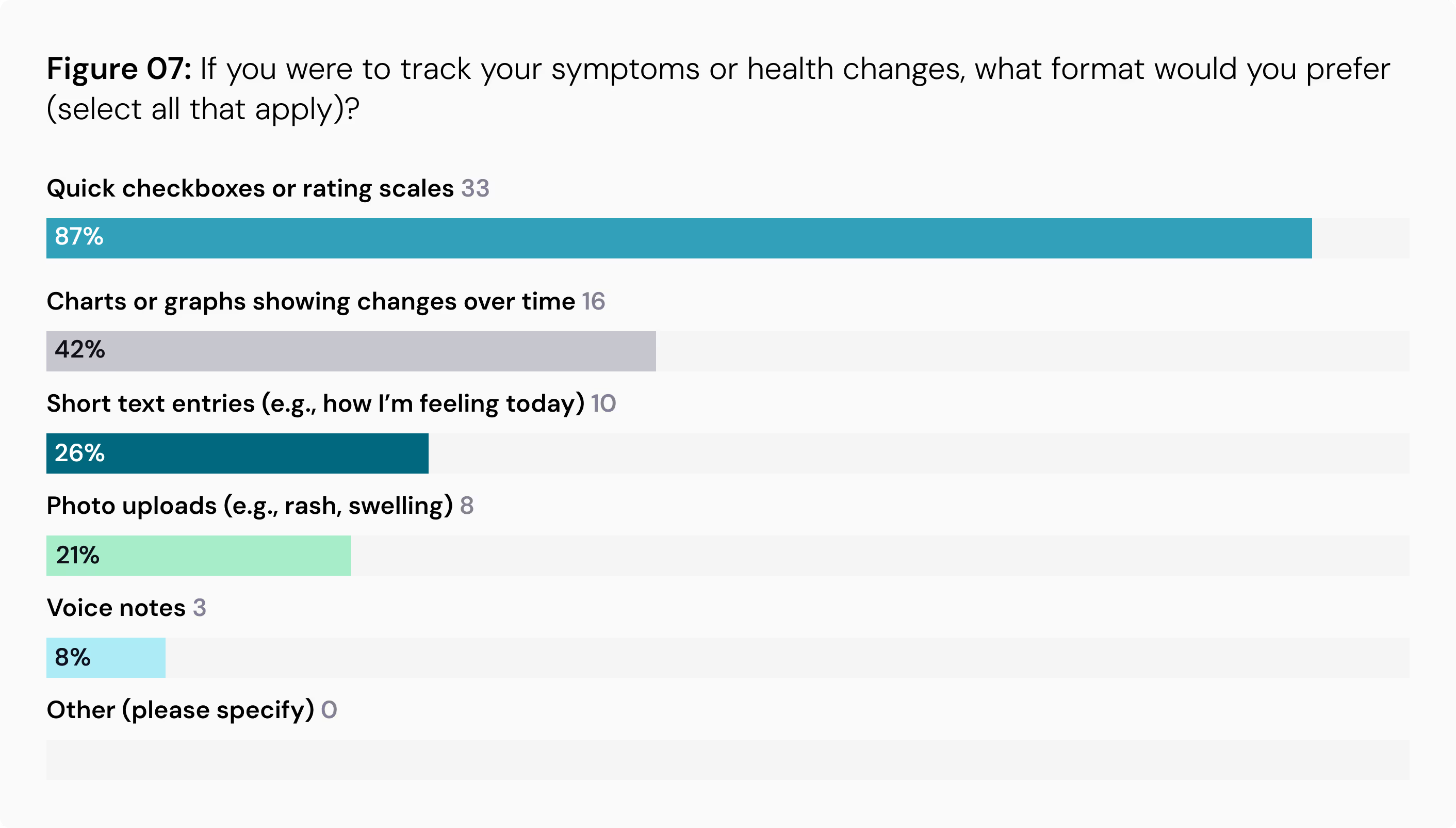
Format Matters: When it comes to how to log symptoms and record in health diaries, simplicity is key. An overwhelming 87% prefer quick checkboxes or rating scales over more demanding formats like photo uploads or voice notes.
The Path Forward
These findings present a compelling case for the widespread adoption of remote data capture in non-interventional research. By embracing these innovations, the research community can generate richer real-world evidence, enhance the patient experience, and lower geographical barriers to participation. As regulatory bodies like the FDA and EMA continue to support the use of digital health technologies, the path is clearing for a more patient-centric and efficient approach to clinical research.
You can find a more detailed look at PicnicHealth’s assessment types in our recent article or contact us.
Data with Dan
High-Density Data: The Foundation for Minimizing Bias in Non-Interventional Research
Non-interventional studies can answer meaningful, complex research questions, but the fragmented U.S. healthcare system creates challenges for capturing the data needed to generate reliable evidence. Methods that rely on real-world data from siloed sources often result in incomplete clinical datasets with missingness that introduces biased research outcomes. This is evident in site-based chart abstractions, which are limited to care received within that site or network, and aggregated EHR datasets, which are anchored in a specific environment with only contributing partners. As a result, these datasets provide limited visibility into all points of care along the patient journey that occur outside of those systems.
Advances in data retrieval technology are now making it possible to capture more complete patient journeys that address these limitations. Once just a technical consideration, the method of data retrieval has become the foundation for generating reliable real-world evidence based on curated, comprehensive data.
Record Availability & Journey Representation
Patient-mediated medical record retrieval and multi-site data retrieval are two methods enabling more complete patient journey capture. Patient-mediated retrieval allows researchers to request medical records on behalf of the patient with their consent, without adding burden to them. Multi-site automated retrieval continuously gathers information from across all healthcare encounters by pulling from both electronic data networks, such as health information exchanges, and direct-to-facility record requests, regardless of EHR vendor or payer coverage.
When used in combination, these methods yield greater visit density for every patient, resulting in greater data density and breadth. Both are critical dimensions of data completeness, ensuring that complete data on key exposures, outcomes, and covariates are captured from the full spectrum of a patient’s healthcare interactions.
Greater Data Density Starts With Greater Visit Density
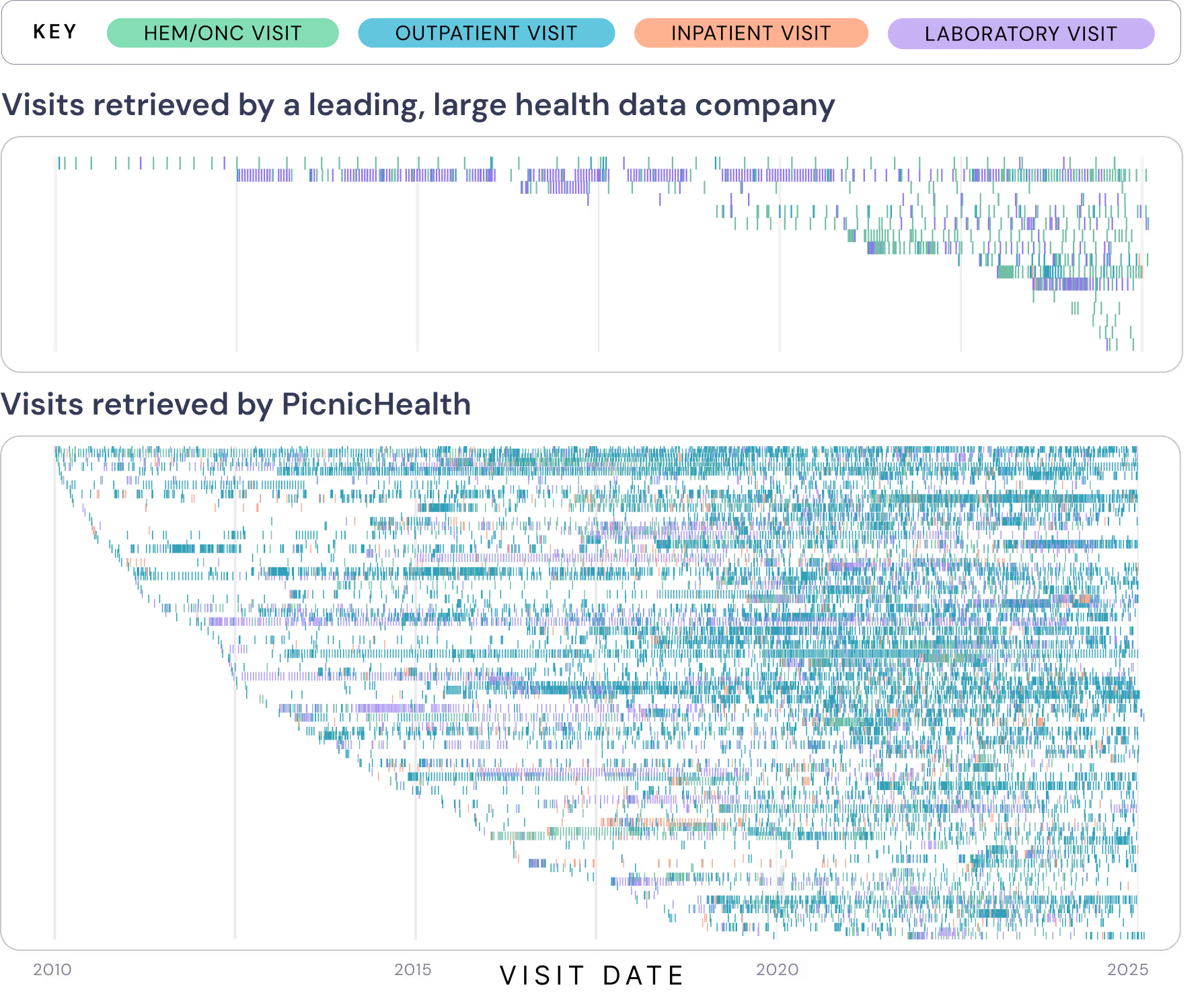
However, not all “advanced data retrieval” methods produce the same degree of data completeness. Compared to a large health data platform company, PicnicHealth’s advanced, best-in-class retrieval method demonstrates greater visit density with respect to time, frequency, and types of routine care settings. This provides a more longitudinal, continuous, and complete medical history for each patient enrolled in the study.
Figure 1: Compares PicnicHealth’s data retrieval to a large health data company’s retrieval for patients with paroxysmal nocturnal hemoglobinuria
Reducing Bias and Confounding Through Completeness
More complete data reduces the risk of selection bias, information bias, attrition bias, and confounding, all of which can undermine the validity of non-interventional research.
Selection bias often arises when data is only collected from specific sites or EHR systems, limiting the diversity of the patient sample. For example, if medical records are only pulled from dermatology clinics, researchers may overrepresent severe cases while missing patients with milder conditions who are treated by primary care providers. Advanced retrieval methods that capture records across multiple care settings ensure a more representative patient population and improve the generalizability of findings.
Information bias occurs when exposures or outcomes are misclassified due to missing data. This may happen when critical study variables, such as treatments, diagnostic biopsy, or emergency room visits, are recorded across multiple provider records at different timepoints. Automated, longitudinal retrieval mitigates this by:
- Continuously collecting data from every provider a patient sees
- Validating and cross-referencing information to ensure accuracy critical to determine eligibility
- Adjusting for risk factors and confounders
- Ascertaining whether or not a clinical outcome occurred
Attrition bias and residual confounding are also mitigated as more complete longitudinal data provides continuity and fills in missing variables. Learn more about how attrition bias is reduced and confounding is addressed in the full whitepaper “Reducing bias in non-interventional research with greater visit and data density.”
Strengthening Robust Non-Interventional Research
Greater visit density, achieved through advanced medical record retrieval that combines patient-mediated and comprehensive multi-site approaches, enables researchers to collect more complete and reliable datasets. This, in turn, improves study design through stronger baseline adjustments, exposure classification, and outcome ascertainment while reducing the risk of bias and missingness that can compromise non-interventional research.
View our latest whitepaper, “Reducing bias in non-interventional research with greater visit and data density,” for a deeper look into how advanced data retrieval methods unlock more reliable real-world evidence.
Break Barriers, Drive Breakthroughs: Expand Your Non‑Interventional Research with AI
Tuesday, October 21st 9:00 - 10:00 AM PST / 12:00 - 1:00 PM EST
Explore how PicnicHealth and our life sciences partners are using AI-powered methodologies to transform non-interventional studies, demonstrating how technology enables more accessible research that captures complete patient journeys and generates robust regulatory-grade insights.

PicnicHealth in the News

Maestro AI featuring Troy Astorino



Clinical Trial Vanguard featuring Rachael T. Higgins

Applied Clinical Trials featuring Troy Astorino

The Tech Trek featuring Troy Astorino

2025 MedTech Breakthrough Awards


Life Sciences Today Podcast featuring Troy Astorino
PicnicHealth’s Latest Publications

Employee Spotlight
Ruby Maa, Product Lead, Data Capture
Please share what your role is at PicnicHealth.
My role is to shape and build PicnicHealth’s data capture product, encompassing data retrieval, abstraction, data quality review, and delivery to our life sciences customers.
Can you share your background prior to joining PicnicHealth?
I have always been interested in data and healthcare. In college, I studied chemical engineering and researched new ways to deliver drugs for gastrointestinal problems to minimize patient side effects. Since then, I have worked at multiple companies across healthcare data and AI/data products, including athenahealth, where I built electronic health record (EHR) software, and most recently Verantos, where I built a natural language processing engine to extract patient data from EHR narratives for clinical research.
What does your day-to-day at PicnicHealth look like?
On any given day, I’m connecting with patients and advocacy organization leaders about what is happening in their communities and bringing those insights back to our team to enhance our platform and inform research with our partners. I also work with our partners to understand their challenges answering key research questions for new treatments and therapies and problem solve solutions for them. When I’m not on a call, I’m researching the feasibility of engaging new communities or specific patient populations for studies on the PicnicHealth platform, and collaborating with our incredible recruitment and enrollment team to understand the impact we’re having on patients as we roll out new campaigns.
FUN FACT: One of my research projects in college involved using multiple high-speed cameras to capture the aerodynamics associated with flushing toilets to understand ways to minimize the spread of bacteria and viruses.
In Case You Missed It
71% of Americans Want Better Medical Record Access—And What It Means for Your Research
A new nationwide survey of more than 1,000 Americans reveals that 71% want a better way to organize their medical records.1 The fragmentation of records has a real impact on patient care: Nearly a quarter of Americans struggle to access records from past providers, while another 22% struggle to understand medical terminology in their own files. One in five has health information scattered across multiple portals, 18% report incomplete records from providers, and 18% say it takes too much time to gather records for appointments.1
This is not just an inconvenience, it is a fundamental barrier to health management and impacts life sciences research. When patients cannot understand their conditions or access their complete medical history, they struggle to effectively advocate for their care, make informed decisions, or manage chronic conditions. When researchers cannot access complete patient records, endpoints could be missed and research conclusions may be biased.
By choosing to work with PicnicHealth, you are not just accessing research participants and more complete insights. You are also providing a solution to one of healthcare's most fundamental problems.
Empowering Patients & Research
Addressing patients' fundamental need for organized, accessible health records, your studies create the foundation for more meaningful research participation. This patient empowerment translates directly into research benefits: comprehensive longitudinal data, engaged participants who understand their conditions, and insights that traditional approaches cannot capture.
When patients have unified access to their medical records and access to our AI health assistant, they gain something missing from their care journey—a clear, complete view of their health and the help they need to understand it. Complex medical terminology becomes digestible. Scattered information across multiple portals becomes organized and actionable.
This creates a fundamentally different dynamic than traditional non-interventional research. Patients are not just participants but empowered partners who can engage meaningfully with their health data and research participation.
The Strategic Advantage
The results of our approach are evident in your studies. Patients who understand and engage with their conditions deeply provide richer insights and contribute perspectives that traditional research approaches miss.
While other research approaches extract data from patients, our model gives back. This mutually-beneficial relationship creates:
- Richer data and ongoing insights
- Higher response rates on patient-reported outcomes and surveys
- Better retention through genuine patient value
- Access to patient perspectives traditional sources miss
- A platform that patients actively want to use
The 71% of Americans seeking better medical record organization represent millions of people struggling to manage their health with inadequate tools. Your studies do not just generate data and inform non-interventional research—they empower patients with the health management tools they desperately need and create a research ecosystem focused around partnership.
Thank you for choosing and trusting us with your research!
1 Researchscape Omnibus Study, August 1, 2025, n= 1083 US adults age 18+
